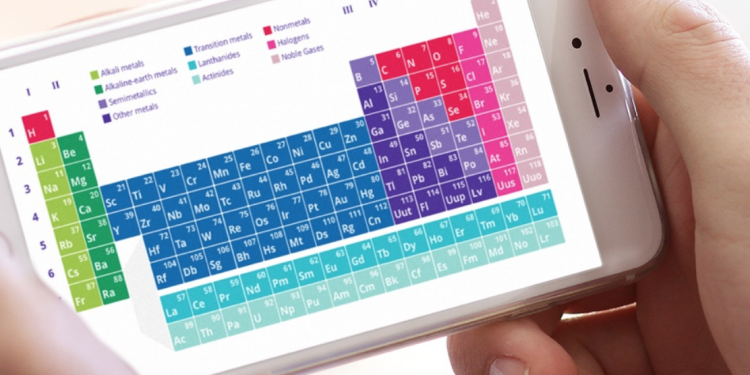
The periodic table of elements is a chart that many of us first saw in science or chemistry class. This table groups all 118 chemical elements in an easy reference format. The elements are arranged based on various trends such as their atomic radius or electron affinity. The table also offers valuable information about each element in a simplified manner.
The Best 10 Apps for the Periodic Table of Elements
The History of the Periodic Table
The development of the periodic table we know today was the result of many years of research by chemists. It started during the late 18th century. Initial attempts of putting together a useful group of elements didn’t follow the table format we are familiar with today. Early attempts by French chemist Antoine Lavoisier in 1789 had the elements in 4 categories, metals, non-metals, earths, and gases. Several years later, a German chemist named Johann Dobereiner came up with a more organized system which consisted of grouping the elements into triads.
When additional chemical properties like valency were discovered, chemists were able to create a new grouping system. This led to organizing the elements in a telluric helix as proposed by French geologist, Alexandre-Émile Béguyer de Chancourtois.
Tabular arrangements of the elements became more popular as the decades went on. The most widely accepted of these was made by a Russian chemist named Dmitri Ivanovich Mendeleev in 1869. The elements were arranged in rows and columns and allowed for the existence of missing elements that had yet to be discovered.
The periodic table that we are familiar with today was created in 1923 following years of research by American chemist, Horace Deming.
Basic Information Provided On a Periodic Table of Elements
The periodic table offers basic yet essential details about the elements in an easy reference format. Most periodic tables feature the names and symbols in each block. These blocks also include the values for atomic mass and the element’s atomic number. Colors are often used to help identify element groups like metals and non-metals. This makes it very easy for teachers, professors and chemists to glance at the table of elements and gather the quick facts about a particular element without having to search the table mindlessly to get the information they need.
Organization of the Periodic Table of Elements
The elements within the periodic table are organized by the atomic number. They are also grouped into rows or columns based on other chemical properties. These patterns are visible in the modern layout of the periodic table of elements with names. The patterns can be used to show trends in electron affinity, atomic radius or ionization energy. A periodic table with charges may also show the electron configuration and other in-depth details that are important to chemists and others in the same industry.
Periodic table groups offer more details on how the various elements share chemical properties with each other. These groups can be easy to identify as each one corresponds with a different column, color or grouping. Elements that are in the same group will typically share electron configurations. Because of this, they may show the same chemical behavior. They can reveal patterns in other chemical properties including electronegativity or ionization energy.
If you are interested in finding a way to memorize the contents of today’s modern periodic table of elements, there are several useful apps currently available to help you do just that. Whether you need this information for an upcoming high school quiz, or simply want to refresh your memory on the basic facts about the elements, these apps for iOS and Android devices can help you achieve your goals.